| Artículos | https://doi.org/10.21041/ra.v12i3.619 |
Electrochemical re-alkalinization applied to carbonated concrete beams samples under the criteria of three standards
Realcalinización electroquímica aplicada a muestras de concreto carbonatadas bajo criterios de tres normatividades
Realcalização eletroquímica de mostras de concreto carbonatado aplicado sob os critérios de três regulamentações
Josefa
de los Angeles Paat Estrella1
*
![]() ,
Jose Alexandro
Miam Cuevas2
,
Jose Alexandro
Miam Cuevas2
![]() ,
Erick Edgar
Bandala3
,
Erick Edgar
Bandala3
![]() ,
Tezozomoc
Pérez López4
,
Tezozomoc
Pérez López4
![]() ,
Demetrio
Nieves Mendoza3
,
Demetrio
Nieves Mendoza3
![]()
1 Doctorado en Ingeniería, Facultad de Ingeniería Mecánica y Eléctrica, Universidad Veracruzana, campus Xalapa, Veracruz, México.
2 Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Campeche, Campeche, México.
3 Facultad de Ingeniería, Universidad Veracruzana, campus Xalapa, Veracruz, México.
4 Centro de Investigación en Corrosión, Universidad Autónoma de Campeche, Campeche, México.
*Contact author: josapaae@uacam.mx
Reception:
June 15, 2022.
Acceptance: August 30, 2022.
Publication: September 01, 2022.
| Cite as: Paat Estrella, J. A., Miam Cuevas, J. A., Maldonado Bandala, E. E., Pérez López, T., Nieves Mendoza, D. (2022),“Electrochemical re-alkalinization applied to carbonated concrete beams samples under the criteria of three standards”, Revista ALCONPAT, 12 (3), pp. 347 –361, DOI: https://doi.org/10.21041/ra.v12i3.619 |
Abstract
The effect of electrochemical re-alkalinization was evaluated by applying the standards UNE-EN-1504, NACE-SP0107-2007 and NMX-C-553-ONNCCE-2018, on previously carbonated reinforced concrete samples. With the monitoring of the degree of re-alkalinization, through pH and half-cell potential measurements at 7, 14, 21 and 28 days, pH recovery is observed in all cases, obtaining characteristic half-cell potential values in each current application, which confirms the polarization of the steel modifying the thermodynamic condition of the concrete-steel interface and causing chemical changes in the concrete paste. With the NMX-C-553-ONNCCE-2018 standard, the steel was polarized without reaching the overprotection region, avoiding the risk of producing hydrogen and brittleness in the steel.
Keywords:
electrochemical re-alkalinization,
pH,
half-cell potential,
current intensity
1. Introduction
The durability of reinforced concrete structures is considered as their ability to keep their physical and chemical conditions unchanged during their useful life when they are subjected to material degradation, so the structural design of a reinforced concrete building, must stipulate the appropriate measures so that it reaches the useful life established in the project, taking into account the environmental and climatic conditions and the type of building to be built.
The deterioration of infrastructure such as bridges, pipelines, tanks, buildings, canals, ports, storage plants, historical monuments, airports, railways, etc., is a serious problem that currently affects not only the agencies responsible for them, but also it indirectly affects society because these structures are rendered unusable for some time before reaching the end of their established useful life. Therefore, within the priorities in Mexico, are the safety of people, real estate and the protection of the environment.
Techniques such as Electrochemical Re-alkalinization (ERA) and Electrochemical Chloride Removal (ECR), have shown to be promising according to the experiences obtained both in the laboratory and in situ (Weichung, Y., Jiang, JC, 2005).
Electrochemical re-alkalinization has been used on numerous occasions in practical and laboratory applications, however, there is currently no consensus on what the parameters are related to the structure and the technique that allow evaluating its effectiveness over time (Gonzalez, F., 2010). Although this technique has shown its effectiveness on real structures, it cannot yet be considered a routine technique due to the lack of detailed information on some aspects such as the side effects it causes and that in the long term it could affect the durability of concrete (Mietz, J., 1998; Rincón, T., 1994).
The application of these electrochemical techniques (ET) as unconventional intervention and maintenance methods have aroused great interest in the field of Civil Engineering. In some of the industrialized countries there are experiences of implementation of the techniques, however, the number of reported works explaining the application conditions is not abundant (Rincón, T. 1994; Helene, P. 1994; Pollet, V. 2000; Bize, B. 2001; Raharinaivo 1992; Chatterji, S. 1994; Fajardo, G., et al. 2006).
As a rehabilitation method or as a preventive treatment, ERA method has been used to recover the alkalinity of concrete near the reinforcement region (Mietz, J., 1998). The advantage of this method is that, when the treatment is finished, the system can be disassembled and the structure of concrete can continue its function without major destructive interventions such as it happens in conventional patch repair treatment (Redaelli, E., & Bertolini, L. 2011).
Studies on the re-alkalinization treatment that focused on understanding the characteristics of the phenomenon, such as the transport mechanisms involved (Aguirre, A., and Gutiérrez, R., 2018; Castellote, M., et al., 2003; González, F ., 2010), the re-passivation efficiency of the reinforcement (Redaelli, E., and Bertolini, L. 2011; Yeih, W., and Chang, J., 2005), the efficiency of different electrolytes in re-alkalinization (Mietz, J. 1995), the effects on the properties of concrete and the side effects (González, F., 2010; Ribeiro, P., et al., 2013; Tong Y., et al., 2012), have been carried out in recent years.
In situ applications were introduced in the late 1980s and a significant number of structures have been treated with this technique. Some documents report on these experiences and show the ability of the technique to recover protective pH levels.
These studies also show that, even after a few years, the alkalinity remains at high levels, which would be sufficient to protect the reinforcement (Yeih, W., Chang, J., 2005). Some of these advances have only been included in local and regional regulations and standards.
As is known, application of an intensity of electric current induces the polarization of the concrete-steel interface with variation in the value of the half-cell potential (HCP), as well as changes in the chemical composition of the concrete paste, mainly reflected by the pH, it was considered convenient to compare 3 re-alkalinization conditions established in the UNE EN 1504, NACE SP0107 2007 and NMX-C-553-ONNCCE-2018 standards, in which the experimental set-up is practically the same, the variation is the application time, being 7 days for the NACE, 14 for the UNE and 28 for the NMX and the applied current intensity, being 4 A/cm2 in the first case, 2 A/m2 for the second and 1 A/m2 for the third, considering the surface of the steel rod.
It is to be expected that the most intense polarization was reached for the NACE conditions, followed by the UNE (EURO) and finally the least for the NMX. Similarly, the rate of re-alkalinization was expected in the same order. However, it was decided to take the polarization up to 28 days in all cases, with the interruption for measurement of carbonation recovery and half-cell potential at 7, 14, 21 and 28 days, to observe the alkalinity recovery capacity and the condition of the concrete-steel interface. At the same time, was recorded the range of HCP values that exceed overprotection values that reach the hydrogen embrittlement zone, a very harmful phenomenon, especially for post-stressed and pre-stressed steel. Results obtained show that, for the NACE and UNE standards, re-alkalinization is achieved in less time, but the HCP values polarize the steel-concrete interface up to the hydrogen generation region, which can lead to the fragility of the metallic element, being a negative factor in the re-alkalinization treatment. With the NMX-C-553-ONNCCE-2018 standard, re-alkalinization occurs more slowly, but it does not reach half-cell potentials in the hydrogen generation zone, so its application under this criterion is more recommended.
2. Experimental procedure
2.1 Materials
Materials used for the manufacture of the concrete specimens complied with current regulations. Aggregates were selected in compliance with NMX-C-111-ONNCCE-2018. Washed sea sand was used as fine aggregate, and crushed stone with a maximum size of 19 mm as coarse aggregate. Materials used are characteristics of the southeastern region of Mexico, whose properties are presented in Table 1.
| Table 1. Characteristics of the components used in the mixtures. | ||||||||||||||
| Tests | Coarse Aggregate | Fine Aggregate | Cement | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSSS (Kg/m3) | 1241 | 1335 | 1400 | |||||||||||
| PLWHA (Kg/m3) | 1417 | 1514 | ||||||||||||
| Density or specific weight (gr/cm3) | 2.26 | 2.56 | 3.2 | |||||||||||
| % Absorption | 2.5 | 2.56 | - - - | |||||||||||
| TMNA (mm) | 12.5 | 2.5 | - - - | |||||||||||
For the manufacture of the specimens, Portland Cement Compound of Rapid Resistance (CPC 30R) was used, with a specific weight of 3200 kg/m3 (NMX-C-414-ONNCCE-2017).
Commercial purified water was supplied with, in order to avoid sample contamination.
2.2 Specimen design and preparation
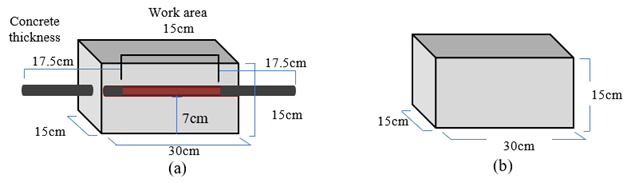
66 concrete specimens were made with reinforcing steel A615 (ASTM), of 0.95 cm (3/8'') and 18 without steel (Figure 1),with a w/c ratio of 0.6, in order to obtain concrete specimens with high porosity to be able to control the diffusion of ions and CO2.
Rods were cut to a length of 50 cm, later they were cleaned with a 1:1 HCl solution, and 17.5 cm from each end were covered with insulating tape, leaving a central work area of 15 cm, as is shown in Figure 1(a).
 |
||||
| Figure 1. Characteristics of the elaborated samples, (a) With a 50 cm rod, with 20 cm uncovered as a work area; (b) Without rod. | ||||
Mix design was carried out as established of ACI (American Concrete Institute) 211.1 for an average compressive strength of 250 kg/cm2.
To improve the plasticity of the mixture, 35 mL/L of a water-reducing fluidizer were used. Amounts of the materials for the mix design are presented in Table 2.
| Table 2. Material weights per cubic meter of concrete mix. | ||||||||||||||
| Materials | Amount used (kg) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | 115.82 | |||||||||||||
| Cement | 288.43 | |||||||||||||
| Coarse aggregate | 500.15 | |||||||||||||
| Fine aggregate | 511.51 | |||||||||||||
After 24 hours, the samples were removed from the mold and subjected to curing by immersion with a saturated solution of Ca(OH)2 for 28 days. After this time, the samples reached an average compressive strength of 204 kg/cm2.
Samples were divided in three series (I, II and III), to which current intensities were applied regarding the steel area in accordance with regulations NMX-C-553-ONNCCE-2018 (1 A/m2), UNE EN 1504 (2 A/m2) and NACE SP0107 2007 (4 A/m2), respectively.
2.3 Accelerated Test
In order to achieve greater carbonation in less time, the specimens were placed in an accelerated carbonation chamber, because carbonation under natural conditions is a slow phenomenon from a technical point of view. During the accelerated tests, the concrete samples were subjected to an environment with a concentration of CO2 of 4 + 0.5%, according to experimental conditions carried out by Turcry, Oksri-Nelfia, Younsi, & Aït-Mok in 2014, and with a relative humidity of 60 + 5%. It has been proposed that with accelerated tests at CO2 concentrations of 4%, the same depth of carbonation than normal concentrations (0.03%) in one year (Moreno, M., et al, 2004).
2.4 Electrochemical Re-alkalinization (ERA)
Electrochemical re-alkalinization is a technique used to recover the alkaline pH from concrete, allowing the re-passivation of the reinforcing steel. The operation of the ERA is very similar to the impressed current cathodic protection, since a continuous electric current is applied from the anode, closing the circuit using the reinforcing steel as the cathode. This technique was carried out based on the criteria established in the NACE SP0107-2007, UNE-EN-1504 and NMX-C-553-ONNCCE-2018 standards, considering the specifications presented in Table 3.
| Table 3. Experimental conditions. | ||||||||||||||
| Parameters | Mexican Standard | European Standard | Nace | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current density | 1 A/m2 | 2 A/m2 | 4 A/m2 | |||||||||||
| Voltage range | 15 – 30 V DC | |||||||||||||
| Anodic system | Galvanized steel mesh | |||||||||||||
| Electrolyte | Sodium Carbonate | |||||||||||||
| Time | 7 - 28 days | |||||||||||||
| pH | Re-alkalinization depth measurement | |||||||||||||
After the accelerated carbonation to which the concrete samples were exposed, they were subjected to the electrochemical re-alkalinization process, requiring the implementation of re-alkalinization device, as described below.
To submit to ERA, 27 samples were selected. Were painted the cross sections of samples for the sodium carbonate penetrated only through the sides of the beam. Subsequently, each of the samples was covered with a galvanized steel mesh, which served as an anode during the RAE, they were arranged vertically in a plastic container with enough capacity, to which a hole was drilled in the base, to expose one side of the rod and not be affected by the immersion electrolyte (sodium carbonate 1M) (see Figure 2).
 |
||||
| Figure 2. Experimental setup used for electrochemical re-alkalinization. | ||||
2.4.1 Measurement of re-alkalinization depth and re-alkalinization current intensity
A parallel connection was made at 3 different currents: 1 A/m2, 2 A/m2 and 4 A/m2, making the connections in triplicate. To carry out this connection, it was necessary to design an electrical distribution device that would maintain a constant electrical flow connected to two power sources to reach the required voltage.
ERA was carried out for 28 days, the samples being divided into three series (serie I, serie II and serie III), of 9 samples each, with the aim of comparing the possible side effects that could occur.
During this time, the pH at the concrete-steel interface and the concrete paste was monitored by the indicator method. These measurements were made every seven days, from the day the ERA began until completing 28 days, as mentioned by several authors (Ton, Y., et al 2012, Yeih, W., and Chang, J., 2005). The extraction of nuclei and the dust samples were carried out on the 15 cm of uncovered rod, using a 1.5” diameter and 7 cm long hollow drill coupled to a drill. (Figure 3). During extraction, depth and pH of each beam exposed to re-alkalinization were determined by the wet method using phenolphthalein (vary between 8.2 -10) and thymolphthalein (vary between 9 -10.5) as indicators. Measurement was made by obtaining an average of the carbonation front from the surface of the sample. Additionally, pH was determined at the depth of the surface of the rod in samples extracted from the samples, by means of the potentiometric method, which determines the level of alkalinity of the concrete, by means of an extraction with distilled water, established in ASTM D4262 -05 (2018) Standard Test Method for pH of Chemically Cleaned or Etched Concrete Surfaces.
 |
||||
| Figure 3. Extraction of cylinders and dust samples. | ||||
2.4.2 Half-cell potential (HCP)
The monitoring of the thermodynamic surface condition of the reinforcement is mainly based on measurements of the HCP, which is related to the active or passive state of the reinforcing steel. The measurement consisted in the determination of the electrical potential difference between the reinforcing steel and a reference electrode (Cu/CuSO4) placed on the concrete surface (American Society for Testing and Materials, 2016), ASTM C-876-15, NMX-C-495-ONNCCE-2015.
3. Results
Next, the experimental results of the electrochemical re-alkalinization of reinforced specimens are presented with a depth of carbonation of 6.7 cm. Results of pH and carbonation coefficient are presented. In the ERA, the behavior of the HCP of the reinforcing steel (cathode) for 28 days, with measurement intervals of 24 hours is shown. Measurements without interrupting the electrical current were made.
3.1 Carbonation
3.1.1 Accelerated carbonation chamber test (ACC)
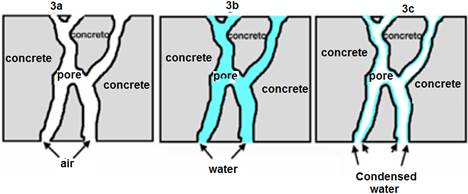
Chemical degradation process or deterioration of concrete by CO2 was carried out over a period of six months. Figure 4 shows the measurement of carbonation progress with respect to time, using the acid-base indicators in accordance with the NMX-C-515-ONNCCE-2016 standard. A linear trend can be seen in the advance of carbonation over the course of the exposure time because there is a great difference between the rate of diffusion of CO2 in air and water, the latter being approximately 104 times lower than in air, so that in the conditions of relative humidity of the ACC (60%) , concrete pores are partially filled, which allows CO2 to diffuse easily. In contrast, if the pores were filled with water (Figure 5) there is hardly any carbonation because there is very little CO2 diffusion in the water (Del Valle et al 2001).
Hydrated cement paste with a pH of 11.5 to 12.5 acquired a red-violet coloration; however, when it was subjected to the action of CO2, a decrease in pH was caused until reaching values of the order of 8 due to the neutralization, to a greater or lesser degree, of the hydroxyl ions, producing, among others, reaction (1):
Calcium carbonate is formed, incorporating CO2 from the environment.
3.1.2 pH measurement at the concrete-steel interface.
Objective of carbonating the specimens prior to the ERA process was to lower the pH and be able to recover it through re-alkalinization to observe the effects caused by this recovery. The average pH value obtained was 8 with a standard deviation of +0.8, which coincided with what was established by Aguirre, AM, et al (2016) and Ribeiro, PHLC, et al (2013) who determined that the pH of the pore solution in practically carbonated areas ranged between 11.5 and values below 9 (Figure 4). This indicates that from the third month of carbonation, the concrete samples were already carbonated, however, to achieve greater pH recovery, it was decided to obtain pH values below 9.
Figure 6 shows the graphs of pH at the level of reinforcing steel and HCP, showing that during the carbonation process the pH gradually decreased, from a pH of approximately 13 to a pH of 9 in the vicinity of the rod, coinciding with the value of the HCP that shifted its initial value of -120 mV to approximately -700 mV, which according to the standard ASTM C-876-15, these values are below the established value of -350 mV, with a 90% risk of corrosion. Raja and Tetsuya (2010), report a similar follow-up, in which the HCP decreases with the advance of carbonation, starting from -180 to -475 mV vs ECS. They propose that the critical carbonation depth is 80% of the total concrete cover.
3.2.1 Electrochemical Re-alkalinization
The electrochemical re-alkalinization technique is an alternative method based on the application of an electric field to a system formed by steel-concrete within an alkaline solution that functions as an electrolyte. It is considered a temporary technique because it is applied between a few days to a few weeks (Gonzalez, F., et al. 2011).
To start the re-alkalinization process, specimens were selected whose corrosion potentials were between -350 mV and -650 mV, with an average carbonation depth of 6.5 cm and a pH below 9. During the duration of the ERA process, a progressive increase in the depth of re-alkalinization was obtained, which meant a recovery in the alkalinity of the concrete from the contact surface between the electrolyte and the sample.
Below are the results obtained from the ERA in the concrete specimens at three current intensities used.
3.2.1.1 Reinforced samples re-alkalized at 1 A/m2 in accordance with NMX-C-553-ONNCCE-2018
During this treatment, indicator technique was used to follow the progress of the re-alkalinization of the concrete in each structure. This evolution was monitored every 7 days during the 28 days of current application.
In the first 7, 14 and 21 days of re-alkalinization (Figures 7, 8 and 9), the samples showed a slight color change to pink and violet in the first three centimeters of depth, due to the phenolphthalein indicators (it changes to pink at pH 8.2 with color intensifying at > pH) and thymolphthalein (turns violet at pH 9.2 with color intensifying at > pH), respectively. At 28 days of re-alkalinization (Figure 10), an increase greater than 11 was obtained, reaching the objective of electrochemical re-alkalinization. As of day 21, both the internal and external RAE are observed overlapping along the 7 cm depth.
3.2.1.2 Reinforced samples re-alkalized at 2 A/m2 in accordance with the UNE-EN-1504 standard
In Figure 11, a sample is observed after 7 days of re-alkalinization, in which the color change can be observed at the end of the 7 cm depth, however, after 14 days of this process the coloration intensifies, remaining constant on days 21 (Figure 12) and 28 (Figure 13), which, according to the results obtained from the determination of pH by the electrode method, reaches values close to 12.
3.2.1.3 Reinforced samples re-alkalized at 4 A/m2 according to the NACE SP0107-2007 standard
Finally, the samples that were subjected to a current intensity of 4 A/m2, which presented an increased coloration from the first 7 days of the re-alkalinization process in a homogeneous way throughout the 7 centimeters of thickness of the concrete (Figure 15), escalating over time and remaining constant until 28 days after re-alkalinization (Figure 16, 17 and 18), reaching pH values above 11(see Figure 19).
In the case of these specimens, an improvement in the propagation of both alkalinities is observed, one produced by the cathodic reaction and the other due to the penetration of the alkaline electrolyte, allowing the effects of the treatment to be extended throughout the depth of the concrete. This coincides with that published by Mietz (1995) and by Redaelli & Bertolini (2011).
According to the intensity of the color, and comparing them with the EURO and NACE standards, the samples under the criteria of the NMX-C-553-ONNCCE-2018 corresponding to 1 A/m2 did not present a considerable increase in pH during the first 7 days of re-alkalinization (see Figure 19), which, according to Aguirre-Guerrero, A., and Mejía de Gutiérrez, R., 2018, makes it less effective in the first 7 days. However, regardless of the regulations used, all the samples at 28 days of re-alkalinization obtained pH greater than 11 (see Figure 19).
As can be seen in Figures 6, 7, 8,9, the coloration of the indicators is presented with greater intensity in two directions, which represents a recovery of pH in the same way, 1) from the reinforcing steel towards the internal surface (internal ERA), due to the production of alkalinity induced by the application of the cathodic current and 2) from the concrete surface towards the reinforcing steel (external ERA) due to the penetration of the alkaline solution in contact with the anodic system (Redaelli, E. & Bertolini, L., 2011), being observed more slowly in the samples subjected to 1 A/m2 (Figure 7).
pH recovery behavior in both directions confirms a production of OH ions induced by the application of a cathodic current (Redaelli, E. & Bertolini, L., 2011, Castellote, M., et al., 2006), and a penetration of the alkaline electrolyte through the concrete (Castellote, M., et al., 2006), confirming that important mechanisms are carried out during the ERA process, such as migration of ions between the magnetic field, negative ions migrate towards the anode (steel mesh), positive ions migrate towards the cathode (reinforcing steel); absorption due to the capillarity effects of alkaline solutions; diffusion of alkaline compounds due to the different concentrations; and electroosmosis of the electrolyte on the concrete surface moving towards the cathode (Redaelli, E. & Bertolini, L., 2011, Castellote, M., et al., 2003, Mietz, 1998 and González, F., et al. 2011).
Regarding the pH results obtained in the three current intensities used (Figure 19), the highest values were presented at higher electrochemical re-alkalinization time.
3.3 Half-cell potential (HCP)
It has been seen that in reinforced concrete structures, the concrete acts as an electrolyte, in this way the reinforcing steel immersed in the cementitious matrix will develop a potential that will depend on the physical and chemical characteristics of the concrete.
Average results of the electrical potentials measured daily during the 28 days of application of the RAE are presented below. Measurements were made in order to obtain a relative value of the probability of corrosion that could have occurred in the reinforcing steel during this electrochemical process.
Samples were subjected to current intensities of 1 A/m2 (NMX), 2 A/m2 (EURO) and 4 A/m2 (NACE) for a period of 28 days, in which the galvanized steel mesh coating was used. as the anode and the reinforcing steel rod as the cathode. During this period, it was observed that the embedded steel maintained half-cell potentials very negative (less than -350 mV), regardless of the applied current intensity, which, according to the provisions of the ASTM C876-15 standard, corresponds to a 90% probability of corrosion. These values indicate that the reinforcing steel remained in an active state throughout the electrochemical re-alkalinization period.
In Figure 20 the behavior of the electrochemical potential that the specimens presented at different intensities of applied current is observed, it can be seen that the HCP values presented a behavior directly proportional to the current intensity that was used, that is, the higher the current intensity, the greater corrosion potential value and vice versa. This current intensity reached values >-900 mV, due to the strong cathodic polarization (Redaelli, E., & Bertolini, L., 2011). Of the three regulations used, it was the test samples under NMX conditions that presented less negative values of HCP. However, in the three cases of current intensities, the steels embedded in the concrete remained active throughout the electrochemical re-alkalinization process. After treatment, the HCP reached values greater than -200 mV, which represents a decrease in the probability of corrosion in accordance with what is established in the standard ASTM C876-15, which, according to Redaelli, & Bertolini, (2011), represents effectiveness in the treatment and suggests that the reinforcing steel reached re-passivation.
It is observed that with the application of the NMX-C-553-ONNCCE-2018 standard, the overprotection region is not reached, which is why it does not represent a risk of producing hydrogen and brittleness in the steel. For the European standard (UNE-EN-1504), the polarization reaches the range of overprotection during the first 12 days, subsequently it increases its HCP values outside the risk zone of hydrogen embrittlement. In these cases, it is convenient to carry out tensile tests to verify if the steel was affected by the generation of hydrogen. It is particularly important to consider in concrete with post-stressed or prestressed steel.
4. Conclusions
The action of CO2 on the hydrated compounds of Portland cement produces a decrease in pH, modifying the chemical composition of compounds and forming various carbonated compounds.
In the application of the electrochemical re-alkalinization technique, the recovery of the pH (re-alkalinization) of the concrete is obtained mainly during the first 7 days at 4 A/m2, allowing a complete recovery of the thickness of the concrete.
However, the stable value or the slight increase in pH together with the increase in the alkali content in the steel-concrete interface would support the conditions that promote the formation of the passive layer of steel that will serve as protection after applying the ERA. Therefore, electrochemical re-alkalinization can be applied as a preventive technique in partially carbonated concrete structures.
A recovery of the pH of the concrete directly proportional to time was achieved, so that of the three regulations used, the specimens under conditions established in the NMX-C-553-ONNCCE-2018 were the ones that presented less negative values of Emc without reaching the overprotection region, which does not represent a risk of producing hydrogen and brittleness in the steel.
5. Acknowledgements
The authors thank the Universidad Veracruzana, Xalapa campus, the Autonomous University of Campeche, the Program for the Development of Teaching Personnel (PRODEP) and the Pablo García Foundation for the facilities provided to carry out this project.To Dr. Victor Moo, for his valuable support in the design and development of the electrical device used during electrochemical re-alkalinization.
References
Aguirre-Guerrero, A. M., & de Gutiérrez, R. M. (2018), Efficiency of electrochemical realkalisation treatment on reinforced blended concrete using FTIR and TGA. Construction and Building Materials, 193, 518-528. DOI: 10.1016/j.conbuildmat.2018.10.195
Aguirre-Guerrero, A. M., Mejía-de-Gutiérrez, R., Montês-Correia, M. J. R. (2016), Corrosion performance of blended concretes exposed to different aggressive environments. Construction and Building Materials, 121, 704-716. DOI:10.1016/j.conbuildmat.2016.06.038
Annual Book of ASTM Standards (2016), Construction. Chemical-resistant materials; vitrified clay, concrete, fiber-cement products; mortars; masonry. Section 4. Vol. 04.05.
Andrade, C., Alonso, C., Rodríguez, J. (1989), Remaining service life of corroding structures. IABSE Symposium on Durability, Lisbon, Sep., pp. 359-363.
ASTM C876-15. Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete.
Bize, B. (2001), Béton armé corrode: Les traitement électrochimiques. In: CSTB Magazine, No. 136. juillet - aoùt 2001.
Castellote, M., Llorente, I., Andrade, C. (2003), Influence of the external solution in the electroosmotic flux induced by realkalisation. in: Mater. Construcc., vol. 53, no 271- 272. pp. 101-111. DOI: https://doi.org/10.3989/mc.2003.v53.i271-272.294
Castellote, M., Llorente, I., Andrade, C., Turrillas, X., Alonso, C., Campo, J. (2006), In-situ monitoring the realkalisation process by neutron diffraction: electro-osmotic flux and portlandite formation. Cem Concr Res. 36:791-800. https://doi.org/10.1016/j.cemconres.2005.11.014
Castellote M., Llorente, I., Andrade C., Turrillas, X, Alonso, C., Campo, J. (2006), Neutron diffraction as a tool to monitor the establishment of the electro-osmotic flux during realkalisation of carbonated concrete. Physica B. 385- 386:26-528. https://doi.org/10.1016/j.physb.2006.05.263
Chatterji, S. (1994), Simultaneous chloride removal and realcalisation of old concrete structures. Cement and Concrete Research 24. No. 6. pp. 1051 -1054. DOI:10.1016/0008-8846(94)90028-0
CYTED - DURAR. Manual de Inspección, Evaluación y Diagnóstico de Corrosión en Estructuras de Hormigón Armado. Reporte Final, Red Durar, CYTED, Maracaibo, (1997).
Fajardo, G., Escadeillas, G., Arliguie, G. (2006), Electrochemical chloride extraction (ECE) from steel reinforced concrete specimens contaminated from artificial seawater. Corrosion Science 48, pp: 110-125. https://doi.org/10.1016/j.corsci.2004.11.015
Del Valle Moreno, A., Pérez-López, T., Martínez Madrid, M. (2001), El fenómeno de la corrosión en estructuras de concreto reforzado. Publicación Técnica No. 182, Secretaría de Comunicaciones y Transportes, Instituto Mexicano del Transporte, Sanfandila, Querétaro.
González Díaz, F. (2010), Realcalinización electroquímica del concreto reforzado carbonatado: una opción de prevención contra la corrosión. Doctorado thesis, Universidad Autónoma de Nuevo León.
González, F., Fajardo, G., Arliguie, G., Juárez, C. A., Escadeillas, G. (2011), Electrochemical Realkalisation of Carbonated Concrete: An Alternative Approach to Prevention of Reinforcing Steel Corrosion. International Journal of Electrochemical Science. 6. pp 6332 - 6349.
Helene, P., Monteiro, J. (1994), Can local repairs be durable solutions for steel corrosion in concrete structures. Annals of international Conference on Corrosion and Corrosion Protection of Steel in Concrete, Vol. 2.
Linares, D., Sánchez, M. (2003), Construction, operation and performance of a chamber for tests of accelerated carbonation. Rev. Tec Ing. Univ Zulia, 26, 34-44.
Mietz, J. (1995), Electrochemical realkalisation for rehabilitation of reinforced concrete structures. Materials and corrosion. 46(9), 527-533. https://doi.org/10.1002/maco.19950460904
Mietz, J. (1998), Electrochemical rehabilitation methods for reinforced concrete structures a state of the art report. EFC N°24, IOM Communications Ltd, London.
NACE (2007), SP0107-2007 Electrochemical Realkalization and Chloride Extraction for Reinforced Concrete
Normas mexicanas del ONNCCE (2018), NMX-C-111-ONNCCE-2018 Industria de la Construcción-Agregados para concreto hidráulico-Especificaciones y métodos de ensayo.
Normas mexicanas del ONNCCE (2017), NMX-C-414-ONNCCE-2017 Industria de la Construcción - Cementantes Hidráulicos - Especificaciones y Métodos de Ensayo.
Normas mexicanas del ONNCCE (2015), NMX-C-495-ONNCCE-2015 Industria de la Construcción - Durabilidad de Estructuras de Concreto Reforzado - Medición de Potenciales de Corrosión del Acero de Refuerzo sin Revestir, Embebido en Concreto - Especificaciones y Método de Ensayo.
Normas mexicanas del ONNCCE (2018), NMX-C-553-ONNCCE-2018 Industria de la construcción - Concreto - Durabilidad - Métodos Electroquímicos de Rehabilitación (Intervención) Especializados (Realcalinización y Remoción de Cloruros) - Especificaciones y Métodos de Ensayo
Norma ACI 211.1. Diseño de mezcla de concreto patrón.
Pollet, V., Dieryck, V. (2000), Re - alkalization: specification for the treatment application and acceptance criteria. Annual Progress Report, 1999 - 2000, COST 521, Workshop, Belfast, p. 271.
Raharinaivo and Carpio, J. (1992). The stepping down the current method: a new corrosion control for cathodic protection of steel. Paper No. 228, NACE Conference Corrosion 92, Nashville USA, p. 9.
Hussain, R. R., Tetsuya, I. (2011), Enhanced electro-chemical corrosion model forreinforced concrete under severe coupled actions of chloride and temperature. Construction and Building Materials Journal. Vol. 25, Issue 3. pp. 1305-1315, Elsevier, ISI. https://doi.org/10.1016/j.conbuildmat.2010.09.014
Redaelli, E., Bertolini, L. (2011), Electrochemical repair techniques in carbonated concrete. Part I: electrochemical realkalisation. J Appl Electrochem 41, 817-827. DOI:10.1007/s10800-011-0301-4
Ribeiro, P. H. L. C., Meira, G. R., Ferreira, P. R. R., Perazzo, N. (2013), Electrochemical Realkalisation of Carbonated Concretes - Influence of Material Characteristics and Thickness of Concrete Reinforcement Cover. Elsevier. Construction and Building Materials 40. 280-290. https://doi.org/10.1016/j.conbuildmat.2012.09.076
Rincón, T., Rincón, O. (1994), Electrochemical evolution of mortar based on acrylic and epoxy resins used to repair concrete structures. 1st Mexican Symposium and 2nd International Workshop on Metallic Corrosion, Mérida, México.
Tong, Y., Bouteiller, V., Marie-Victoire, E., Joiret, S. (2012). Efficiency Investigations of Electrochemical Realkalisation Treatment Applied to Carbonated Reinforced Concret. Part 1: saacrifical anode process. Cem. Concr. Res. 42 (1), 84-94. DOI:10.1016/j.cemconres.2011.08.008
Tuutti, K. (1982). Corrosion of steel in concrete. Report 4.82, The Swedish Cement and Concrete Association, Stockholm.
UNE-EN 1504 Norma de productos y sistemas para la protección y reparación de estructuras de hormigón.
Yeih, W., Chang, J. J. (2005), A study on the efficiency of electrochemical realkalisation of carbonated concrete. Construction and Building Materials 19. 516-524 p. https://doi.org/10.1016/j.conbuildmat.2005.01.006
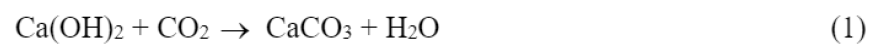
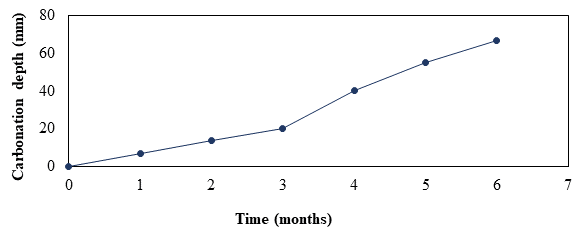
Figure 4.
Carbonation depth with respect to exposure time in CCA.
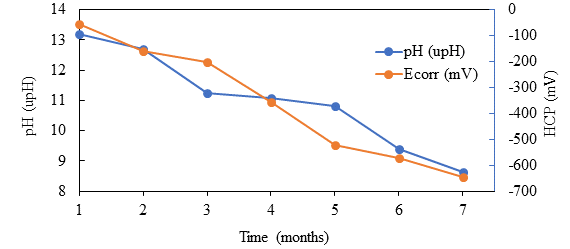
Figure 6.
pH and HCP monitoring during carbonation

Figure 19.
pH values during the 28 days of RAE treatment.
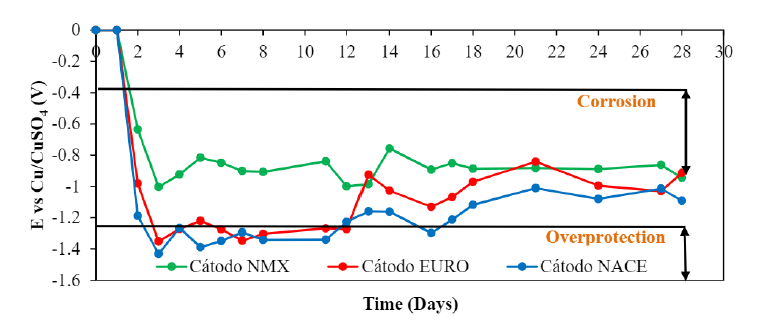
Figure 20.
Average values of half cell potentials (V) during the 28 days of RAE subjected to three different current intensities.