| Basic Research | https://doi.org/10.21041/ra.v11i2.480 |
Procedure to detect the penetration of chlorides into carbonated concrete with silver nitrate
Procedimento para detectar a penetração de cloretos com nitrato de prata em concreto carbonatado
Procedimiento para detectar la penetración de cloruros con nitrato de plata en hormigón carbonatado
C. Vieira Pontes1 2, G. Costa Reus1 *, A. Calvo1 2, M. H. F. Medeiros1 2 3
1 Programa de Pós-graduação em Engenharia de Construção Civil (PPGECC) , Brasil .
2 Universidade Federal do Paraná (UFPR), Brasil.
3 Centro de Estudos em Engenharia Civil (CESEC), Brasil.
*Contact author: giovanacostareus@gmail.com
Reception: April 16, 2020.
Acceptance: February 12, 2021.
Publication: May 01, 2021.
| Cite as: Vieira Pontes, C., Costa Reus, G., Calvo, A., Medeiros, M. H. F. (2021), "Procedure to detect the penetration of chlorides into carbonated concrete with silver nitrate", Revista ALCONPAT, 11 (2), pp. 76 – 88, DOI: https://doi.org/10.21041/ra.v11i2.480 |
Abstract
The main objective of this work is to propose a standard procedure that enables the use of the colorimetric method to measure the depth of chloride penetration during inspections of concrete structures exposed to both chlorides and carbonation. To avoid the occurrence of false positive results, solutions of calcium hydroxide (Ca(OH)₂) and sodium hydroxide (NaOH) were tested as a pretreatment. The tests were carried out on carbonated only samples, and on carbonate and chloride contaminated samples. The results show that the NaOH solution eliminates the carbonation interference. Therefore, a suitable method was found to introduce depth readings of chloride contamination in concrete field inspections.
Keywords:
durability of concrete,
chloride attack,
silver nitrate,
carbonation,
environmental aggressiveness.
Resumo
Este trabalho tem por objetivo principal propor um procedimento padrão para a utilização do método colorimétrico para medir a profundidade de penetração de cloretos nas inspeções de estruturas de concreto em que existe a exposição aos cloretos e à carbonatação simultaneamente. Para evitar a ocorrência de resultados "falsos positivos" foram testadas as soluções de hidróxido de cálcio (Ca(OH)₂) e hidróxido de sódio (NaOH) como tratamento prévio. Os testes foram conduzidos em amostras apenas carbonatadas e em amostras contaminadas por cloretos e carbonatadas. Os resultados mostram que a solução de NaOH elimina a interferência da carbonatação. Desse modo, chegou-se a indicação de um método adequado para introdução de leituras de profundidade de contaminação por cloretos nas inspeções do concreto em campo.
Palavras-chave:
durabilidade do concreto,
ataque por cloretos,
nitrato de prata,
carbonatação,
agressividade ambiental.
Resumen
Este trabajo tiene como objetivo principal proponer un procedimiento estándar que viabilice el uso del método colorimétrico para medir la profundidad de penetración de cloruros durante las inspecciones de estructuras de hormigón expuestas tanto a cloruros como a carbonatación. Para evitar la aparición de resultados "falsos positivos", se probaron soluciones de hidróxido de calcio (Ca(OH)₂) e hidróxido de sodio (NaOH) como pretratamiento. Las pruebas se llevaron a cabo en muestras solamente carbonatadas, y en muestras contaminadas por cloruros y carbonatadas. Los resultados muestran que la solución de NaOH elimina la interferencia de la carbonatación. Por lo tanto, se llegó a un método adecuado para introducir lecturas de profundidad de contaminación por cloruro en inspecciones de estructuras de hormigón en campo.
Palabras clave:
durabilidad del hormigón,
ataque por cloruros,
nitrato de plata,
carbonatación,
agresividad ambiental.
1. Introduction
Corrosion of steel is one of the most common degradation mechanisms in reinforced concrete structures, the main causes of which are carbonation and attack by chloride ions (Corral et al., 2013). Both phenomena are responsible for dissolving the thin layer of iron oxide, a passive layer which covers and protects steel bars against corrosion in environments with a pH above 11 (Helene, 1993; Montemor et al., 2003; Moreira, 2006; France, 2011).
The entry of chloride ions into the concrete occurs at different speeds in the same building, depending on the different microclimates that occur throughout it, as mentioned by Medeiros et al. (2013), Medeiros Junior et al. (2015a) and Medeiros Junior et al. (2015b). As reported by different authors (Helene, 1993; Montemor et al., 2003; Medeiros et al., 2009a; France, 2011; Real et al., 2015), the presence of chloride ions in reinforced concrete is due to the diffusion of these ionic elements from the external environment to the interior of the structure, or the use of contaminated raw materials for the production of concrete.
The attack by chlorides in steel generates an expansive reaction; that is, the chloride ions react with the iron ions in the reinforcement and form products (iron oxides and hydroxides) that have larger volumes than the original iron ions. This phenomenon generates internal stresses that can cause cracks in the structure if they exceed the concrete's tensile strength (Cascudo, 1997; Montemor et al., 2003). In addition, chloride ions corrode the reinforcement locally (in the form of pits), reducing the cross section of the bearing element and affecting its structural function (França, 2011).
Within this context, the penetration of chlorides into concrete structures is a possible cause of reinforcement corrosion, and it is important to know the depth of penetration of this aggressive ion for inspection and diagnosis work of reinforced concrete.
2. Relevance of the theme
To inspect or monitor concrete structures to detect the presence, depth and/or evolution of chloride ion penetration, the colorimetric method of spraying silver nitrate solution (AgNO3) is used (Baroghel-Bouny et al., 2007; Real et al., 2015). The spraying of the silver nitrate solution chemical indicator is a visual colorimetric method of inspection and was originally standardized by UNI 7928 in 1978. It is a qualitative technique of practical application in samples of concrete structures, in addition to being low cost if compared to concrete dust extraction and chloride profile determination in titration or potentiometry procedures (França, 2011; He et al., 2012).
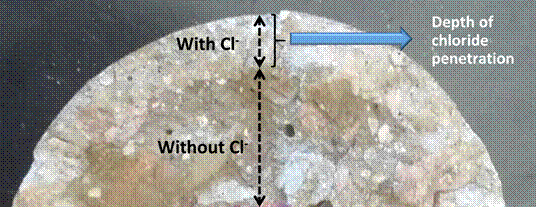
According to Baroghel-Bouny et al. (2007), Medeiros et al. (2009b) and Kim et al. (2013), the technique consists of spraying silver nitrate solution on the recently fractured cross section of concrete cores to form two distinct regions in terms of color: one with brown precipitate corresponding to the region without chlorides, and the other without color change in the region affected by chlorides. Figure 1 shows the colorimetric method of spraying AgNO3 solution as applied to a concrete specimen.
 |
||||
| Figure 1. Chloride penetration depth measurement by visual colorimetric method by spraying an aqueous solution of 0.1 M AgNO3. | ||||
The photochemical reactions after spraying the silver nitrate solution correspond to the combination of silver ions and free chloride ions forming silver chloride (AgCl), which has a whitish color, according to Equation (1). In regions with no free chlorides, there is a photochemical reaction between silver ions and hydroxyl ions forming AgOH, and later Ag2O, which gives the concrete a brown color (Yuan et al., 2008; France, 2011; He et al., 2012; Kim et al., 2013; Real et al., 2015).
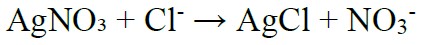 |
(1) |
The colorimetric method of spraying silver nitrate is widely used in experimental works in which the tested concrete is found in saturation conditions and free from the carbonation process. Many works published in recent years in high-impact magazines (such as: Ferreira et al., 2016; Weiss et al., 2017; Wei et al., 2018; He et al., 2018; Slomka-Slupik et al., 2018; Fernández-Ruiz et al., 2018; Lau et al., 2018; Azarijafari et al., 2018) confirm its frequent use in current research.
However, some studies (França, 2011; Real et al., 2015) point out that the silver ions (Ag+) in the silver nitrate solution react with the carbonation product CO3, forming Ag2CO3, which also gives a whitish color to the concrete. Thus, carbonation interferes with the colorimetric method of spraying silver nitrate solution on cementitious materials, generating a possible false positive result, as reported by Medeiros et al. (2018).
Therefore, when inspecting carbonated structures with the colorimetric method of silver nitrate solution, there is an indication of the presence of chloride ions in regions that do not necessarily have chlorides, but rather have carbonates with a pH less than 10. For this reason, the false positive result can invalidate the application of the colorimetric method of silver nitrate solution to cement structures exposed to the environment, since carbonation is a degradation mechanism inherent to constructions exposed to the environment.
In addition, due to the occurrence of large Brazilian demographic densities in coastal areas associated with industrialization processes, it appears that the phenomena of attack by chloride ions and carbonation occur simultaneously in numerous reinforced concrete structures (Real et al., 2015; Medeiros et al., 2013).
Within this context, there is a limitation to the use of silver nitrate solution spraying to detect the front of chloride penetration in real constructions exposed to environments with chlorides and the carbonation process, both simultaneously interacting with the concrete in service conditions. The objective of this research is, therefore, to develop a standard procedure to detect the penetration depth of chlorides in carbonated concrete.
3. Materials and methods
3.1 Materials
The cylindrical concrete specimens used in both phases of the research were dosed with C PV-ARI type cement, with dimensions of 100 mm in diameter and 200 mm in height. For each case of the study, three repetitions were performed, that is, three specimens under the same conditions for each measurement. The results were then averaged.
Table 1 shows the chemical composition of the cement and quartz filler used to provide the materials in the concrete dosage. In addition, the physical characteristics of the cement and quartz filler are shown in Table 2.
| Table 1. Chemical analysis, by X-ray fluorescence, of CP V-ARI cement and quartz filler. | ||||||||||||||
| Binder | Chemical analysis (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | K2O | TiO2 | |||||||
| CP V - ARI | 73.21 | 10.45 | 3.59 | 3.71 | 3.66 | 3.05 | 1.36 | 0.00 | ||||||
| Quartz filler | 0.00 | 95.65 | 2.43 | 0.00 | 0.00 | 1.77 | 0.00 | 0.04 | ||||||
| Table 2. Physical characteristics of CP V-ARI cement and quartz filler. | ||||||||||||||
| Binder | Specific Mass (g/cm3) | BET specific area (m2/kg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C PV-ARI | 3.09 | 1.070 | ||||||||||||
| Quartz filler | 2.60 | 1.227 | ||||||||||||
Natural quartz sand from Balsa Nova, Brasil was used as fine aggregate, which has a specific mass of 2.63 g/cm³, a unit mass of 1.54 g/cm³ and a fineness module of 2.34. The coarse aggregate was basalt, having a maximum dimension of 19 mm and specific gravity of 2.64 g/cm³.
The mixing ratio of the concrete used was 1: 0.10 : 2.25 : 3.00 (cement : quartz filler : fine aggregate : coarse aggregate) with a water/cement ratio of 0.50. The slump was kept constant in the range of 10 ± 2 cm. This concrete presented compressive strength of 43 MPa and its water sorptivity was 0.0059 g/cm².hours0,5.
Before beginning the accelerated carbonation, a seasoning process was adopted, which consisted of the NORIE method as presented in Pauletti (2004). The process consists of arranging the specimens in an air-conditioned room with controlled humidity and temperature. When the weight variation of the specimen is less than 0.10 g in 24 hours, the sample is suitable for the accelerated carbonation test.
The samples were subjected to accelerated carbonation for 12 weeks in a carbonation chamber with a CO2 content of 5 ± 0.5%. The relative humidity inside the equipment was set at 60 ± 1% and the temperature at 40 ± 0.3ºC. The carbonation coefficient of the substrate concrete was 1.96 mm/week0.5. These data agree with those published by Medeiros, Raisdorfer and Hoppe Filho (2017).
3.2 Methods
Carbonated concrete - Chloride-free:
Initially, the validity of sodium hydroxide and calcium hydroxide solutions was analyzed in the pH increase of the concrete surface and subsequent elimination of the false positive result caused by carbonation in the AgNO3 solution spraying method.
For this, carbonated and chloride-free specimens were used. Each specimen was sectioned longitudinally in four slices of the same height for spraying NaOH and Ca(OH)2 solutions. Saturated aqueous sodium hydroxide solution (150.00 g/L) was sprayed onto two slices of the specimen, and saturated aqueous calcium hydroxide solution (1.85 g/L) was sprayed on the other two sections, aiming to test the effectiveness of the solutions. To quickly dry the slices, they were put in a dry chamber with 55 ± 5% relative humidity and temperature at 23 ± 2ºC for about an hour.
After this procedure, a solution of phenolphthalein was applied to one half of the cross section of each quarter of sectioned sample, in the proportion of 5 g of phenolphthalein to 276.15 g of ethyl alcohol to 150 g of distilled water. Silver nitrate solution was sprayed twice in succession on the other half at a concentration of 0.10 mol/L. The application of silver nitrate was repeated to increase the color contrast between the areas with and without chlorides, as was done in the work of Baroghel-Bouny et al. (2007).
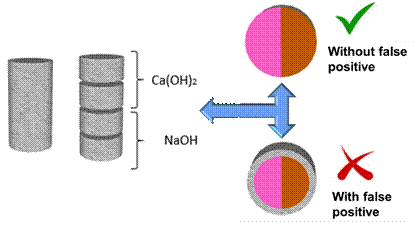
Then, visual evaluation was carried out on the samples. If the fraction with a chemical phenolphthalein indicator acquired a crimson red color throughout, the alkaline solution would have been effective in increasing the pH of the carbonation front and avoiding the false positive. At the same time, the half with AgNO3 solution should change color to brown throughout; that is, it should not indicate the false positive result for the presence of chloride ions because the specimens were carbonated without chlorides. Figure 2 shows the schematic of the idealized experiment.
 |
||||
| Figure 2. Schematic of the experiment to eliminate the false positive result caused by carbonation. | ||||
Carbonated concrete - With chlorides:
Then, the experiment was carried out to measure, with an aqueous solution of silver nitrate, the depth of penetration of chlorides in specimens affected by carbonation and attack by chlorides, simultaneously. The alkaline solution inhibiting the effects of carbonation on the concrete surface was previously applied. For this, three specimens were used for each accelerated chloride penetration time (24h, 48h and 72h), totaling 9 specimens.
To induce the entry of chlorides in the samples quickly, a system based on the migration of chlorides was elaborated. The migration of ions is caused by the difference in electrical potential between the mediums. The positive ions move towards the negative pole and the negative ions move towards the positive pole. According to Medeiros (2008), this movement occurs both through migration and through diffusion. However, migration is more considerable in these test conditions.
For testing this experiment, all specimens were saturated and then immersed in an aqueous solution with 3% NaCl, since migration occurs in a saturated medium. Then, a steel bar and a wire mesh were connected to a 30 V electrical source, inducing the positive pole in the hole inside the sample. In this way, the Clˉ anions, disassociated in the sodium chloride solution, were electrostatically attracted into the specimens. Figure 3 shows a schematic of the apparatus assembled to induce chloride penetration in concrete specimens.
After the chloride immersion/migration period, the concrete elements were dried in an oven at 40°C for 24 hours, cooled for another day and then broken up to be sprayed by the alkaline solutions. The alkaline solution was applied to raise the pH of the carbonation front on the newly fractured surface and prevent the occurrence of the false positive result. After spraying this solution, the test specimens remained in a dry chamber (55±5% and 23±2°C temperature) for 01 hour to eliminate excess moisture on the concrete surface.
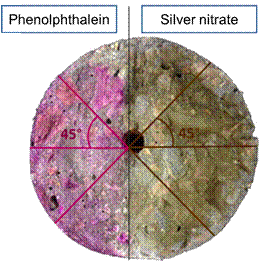
Subsequently, phenolphthalein solution was applied to confirm that the solution had alkalized the surface, and silver nitrate solution was applied to try to measure the depth of chloride penetration. With the distinction of the whitish and brown regions in the half with silver nitrate, it was possible to measure the penetration depth of chlorides from the surface of the element. Measurements were taken with a caliper at five different points from half the cross section, as shown in Figure 4.
 |
||||
| Figure 3. Schematic of the chloride migration test: (a) schematic of the electrical connection; (b) photo of the assembled experiment. | ||||
 |
||||
| Figure 4. Schematic of the locations for measuring the depth of carbonation and the penetration of chloride ions. | ||||
To confirm the depth of chloride penetration with the colorimetric test, the chloride profile was determined by using the RILEM TC 178-TMC sample collection procedure published by Vennesland, Climent, Andrade (2013). Thus, after the chloride migration process, the specimens were dried in an oven at 40°C for 24 hours and cooled by air for another 24 hours. After that, one third of the Ø10 x 20 cm cylindrical specimens was used to collect concrete dust from a drill. The sample collection was performed every 10 mm in depth, up to 40 mm in depth. For each depth of dust collection, the determination of the content of acid-soluble chlorides (total chlorides) was carried out by titration with silver nitrate after an attack with nitric acid, as detailed in ASTM C1152 (2020).
4. Results and discussions
4.1 Carbonated concrete - Chloride-free:
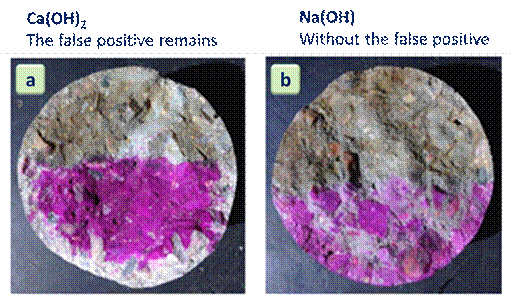
Figure 5 shows the result of the attempt to neutralize the effect of carbonation in the test with silver nitrate using the spray of an aqueous solution saturated with calcium hydroxide and another saturated with sodium hydroxide. All samples were carbonated and free from chloride contamination.
It is observed that the spraying of a saturated solution of calcium hydroxide did not raise the pH of the carbonated concrete layer to the point of reaching the turning point of the phenolphthalein chemical indicator solution. This probably occurred because of the low concentration of hydroxyl in the saturated solution as calcium hydroxide. This is due to the low solubility of Ca(OH)2, as reported by Defendants (2017).
On the other hand, the sodium hydroxide solution increased the basicity of the concrete, which was verified in the visual analysis after spraying the chemical pH indicator and the AgNO3 solution. This result was like that found by Pontes et al. (2020). The NaOH solution was also effective in re-alkalizing concrete samples in the work of Réus (2017) and Réus and Medeiros (2020).
Thus, it can be said that the spraying of solution saturated with sodium hydroxide was effective in eliminating the occurrence of false positive results when an aqueous solution of silver nitrate is sprayed on carbonated concrete that is not contamination by chlorides.
 |
||||
| Figure 5. Carbonated specimens with (a) previous spraying of Ca(OH)2 solution (b) previous spraying of NaOH solution. | ||||
4.2 Carbonated concrete - With chlorides:
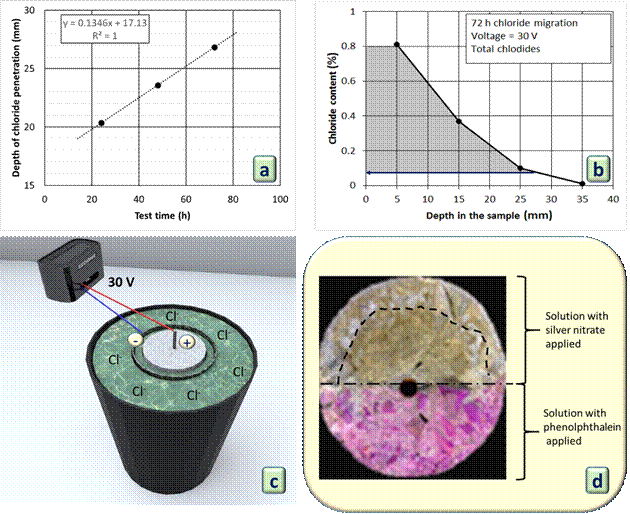
After the immersion of the concrete samples in the sodium chloride solution during the chloride migration test, the penetration depth of chloride ions was measured at the end of the 24-hour, 48-hour and 72-hour test cycles (Figure 6-a). This measurement was performed with the previous application of the sodium hydroxide solution, effective in alkalinizing the carbonated concrete as shown in Figure 5. This procedure was performed to prevent the occurrence of the false positive result that the silver nitrate solution presents in carbonated samples.
The entry of chlorides occurred gradually over the test time and the final depth of chloride penetration in the concrete was 26.8 mm.
Figure 6-b shows the total chloride profile determined in the concrete of this experiment, after 72 hours of migration with 30 V ddp. The data show the coherence of the chloride penetration depth result by the colorimetric method, in which the color change occurred from the chloride content of 0.08% in relation to the cement mass. This comparison was carried out to obtain proof of the coherence of the proposed method for determining the penetration depth of chlorides.
Figure 6-c illustrates the chloride ion migration experiment and Figure 6-d shows a photo of a specimen after applying the colorimetric method. This photo shows a freshly fractured specimen whose surface was previously treated with a NaOH solution spray, after which the AgNO3 solution was applied. Once again, it was observed that the sodium hydroxide solution eliminated the false positive effect generated by the carbonation of the concrete in the silver nitrate spraying method. This is evident, as the region of the samples sprayed with phenolphthalein did not identify the carbonated area. Only the section with AgNO3 solution generated a color change in the regions with the presence of free chlorides, since in this phase the samples were carbonated and contaminated by chlorides.
5. Conclusions
This work presents a possible path for the use of the colorimetric method of spraying silver nitrate solution under service conditions in which reinforced concrete is exposed to chloride attack and to the concurrent carbonation process. The work indicates what can be done before the application of the silver nitrate solution to avoid a false positive, which has prevented the use of this colorimetric method in inspection work on real constructions.
In this context, it is concluded that the step-by-step procedure is effective to enable the use of the colorimetric method with silver nitrate to obtain the depth of penetration of chlorides in concrete in situations where it is exposed to the penetration of chlorides and to the carbonation process.
The steps are:
Step 1 - Part of the concrete piece must be sectioned and a saturated aqueous solution of sodium hydroxide (150 g/L) is applied on the newly fractured surface by spraying. Wait about an hour for the surface to dry;
Step 2 - Spray a solution of silver nitrate twice in succession (5 minutes interval between each time) at a concentration of 0.10 mol/L. Wait about an hour for the surface to dry and the reactions to occur, generating the color contrast. As illustrated in Figure 7, the brown color corresponds to the region without chlorides, and the other without color change corresponds to the region affected by chlorides;
Step 3 - Measure the depth of chloride penetration using a ruler or caliper. Work with average, minimum and maximum values of chloride penetration depth to interpret the results.
6. Acknowledgements
The authors express their gratitude to the Brazilian agencies CNPq, Capes and Fundação Araucária for the scholarship and financial support, to the Federal University of Paraná (UFPR), the Polytechnic Center, the Department of Civil Construction (DCC), the Graduate Program in Civil Construction Engineering (PPGECC), the Civil Engineering Study Center (CESEC), the Materials and Structures Laboratory (LAME) and the Construction Pathology and Recovery Research Group (PRC).
References
ASTM International. (2020). ASTM C1152/C1152M-20 Standard Test Method for Acid-Soluble Chloride in Mortar and Concrete. https://doi.org/10.1520/C1152_C1152M-20
Azarijafari, H., Azarijafari, H., Tajadini, A., Rahimi, M., Berenjian, J. (2018), Reducing variations in the test results of self-consolidating lightweight concrete by incorporating pozzolanic materials. Construction and Building Materials. 166: 889-897. https://doi.org/10.1016/j.conbuildmat.2018.01.121
Baroghel-Bouny, V., Belin, P., Maultzsch, M., Henry, D. (2007), AgNO 3 spray tests: advantages, weaknesses, and various applications to quantify chloride ingress into concrete. Part 1: Non-steady-state diffusion tests and exposure to natural conditions. Materials and Structures. 40: 759-781. https://doi.org/10.1617/s11527-007-9233-1
Cascudo, O. (1997), "Controle da Corrosão de Armaduras em concreto: inspeções e técnicas eletroquímicas". PINI, 1.ed., São Paulo, Brasil.
Corral, R., Arredondo, S., Almaral, J., & Gómez, J. (2013). Chloride corrosion of embedded reinforced steel on concrete elaborated from recycled coarse aggregates and supplementary cement materials. Revista Ingeniería de Construcción, 28(1): 21-35. http://dx.doi.org/10.4067/S0718-50732013000100002
Fernández-Ruiz, M. A., Gil-Martín, L. M., Carbonell-Márquez, J. F., Hernández-Montes, E. (2018), Epoxy resin and ground tyre rubber replacement for cement in concrete: Compressive behaviour and durability properties. Construction and Building Materials. 173: 49-57. https://doi.org/10.1016/j.conbuildmat.2018.04.004
Ferreira, R. M., Castro-Gomes, J. P., Costa, P., & Malheiro, R. (2016). Effect of metakaolin on the chloride ingress properties of concrete. KSCE Journal of Civil Engineering, 20(4), 1375-1384. https://doi.org/10.1007/s12205-015-0131-8
França, C. B. (2011), "Avaliação de cloretos livres em concretos e argamassas de cimento Portland pelo método de aspersão de solução de nitrato de prata". Dissertação de Mestrado, Programa de Pós-graduação em Engenharia Civil, Universidade Católica de Pernambuco, Recife, 85 p.
He, F., Shi, C., Yuan, Q., Chen, C., Zheng, K. (2012), AgNO 3 -based colorimetric methods for measurement of chloride penetration in concrete. Construction and Building Materials. 26: 1-8. https://doi.org/10.1016/j.conbuildmat.2011.06.003
He, F., Shi, C., Yuan, Q., An, X., Tong, B. (2018), Corrosion of cement pastes made of CEM I and CEM III/A caused by a saturated water solution of ammonium chloride after 4 and 25 days of aggressive immersion. Construction and Building Materials. 170: 279-289. https://doi.org/10.1016/j.conbuildmat.2018.03.073
Helene, P. (1993), "Contribuição ao estudo da corrosão em armaduras de concreto armado". Tese de Livre Docência, Escola Politécnica, Universidade de São Paulo, São Paulo, 231 p.
Kim, M-Y., Yang, E-I., Yi, S-T. (2013), Application of the colorimetric method to chloride diffusion evaluation in concrete structures. Construction and Building Materials. 41: 239-245. https://doi.org/10.1016/j.conbuildmat.2012.11.084
Lau, P. C., Teo, D. C. L., Mannan, M. A. (2018). Mechanical, durability and microstructure properties of lightweight concrete using aggregate made from lime-treated sewage sludge and palm oil fuel ash. Construction and Building Materials. 176: 24-34. https://doi.org/10.1016/j.conbuildmat.2018.04.179
Medeiros, M. H. F. (2008), "Contribuição ao estudo da durabilidade de concretos com proteção superficial frente à ação de íons cloretos". Tese de Doutorado, Universidade de São Paulo, São Paulo.
Medeiros, M. H. F., Hoppe Filho, J., Helene, P. (2009a), Influence of the slice position on chloride migration tests for concrete in marine conditions. Marine Structures. 22: 128-141. https://doi.org/10.1016/j.marstruc.2008.09.003
Medeiros, M. H. F., Helene, P. (2009b), Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption . Construction and building materials. 23(3): 1476-1484. https://doi.org/10.1016/j.conbuildmat.2008.06.01
Medeiros, M. H. F., Gobbi, A., Réus, G. C., Helene, P. (2013), Reinforced concrete in marine environment: Effect of wetting and drying cycles, height and positioning in relation to the sea shore. Construction and Building Materials. 44: 452-457. https://doi.org/10.1016/j.conbuildmat.2013.02.078
Medeiros Junior, R. A., Lima, M. G., Brito, P. C., Medeiros, M. H. F. (2015a), Chloride penetration into concrete in an offshore platform-analysis of exposure conditions. Ocean Engineering. 103: 78-87. https://doi.org/10.1016/j.oceaneng.2015.04.079
Medeiros-Junior, R. A., Lima, M. G., Yazigi, R., Medeiros, M. H. F. (2015b), Carbonation depth in 57 years old concrete structures. Steel and Composite Structures. 19(4): 953-966. https://doi.org/10.12989/scs.2015.19.4.953
Medeiros, M. H. F. D., Raisdorfer, J. W., & Hoppe Filho, J. (2017). Influência da sílica ativa e do metacaulim na velocidade de carbonatação do concreto: relação com resistência, absorção e relação a/c. Ambiente Construído, 17(4), 125-139. https://doi.org/10.1590/s1678-86212017000400189
Medeiros, M. H. F., Réus, G. C., Pontes, C. V. (2018), "Nitrato de prata como método colorimétrico para detecção da penetração de cloretos: análise crítica". in: 3° Simpósio Paranaense de Patologia das Construções, 2018, Curitiba., v. único. pp. 35-46. https://doi.org/10.4322/2526-7248.017
Montemor, M. F., Simões, A. M. P., Ferreira, M. G. S. (2003), Chloride-induced corrosion on reinforcing steel: from the fundamentals to the monitoring techniques. Cement and Concrete Composites. 25: 491-502. https://doi.org/10.1016/S0958-9465(02)00089-6
Moreira, C. (2006), "Realcalinização de estruturas de concreto carbonatado com utilização de gel saturado de solução alcalina". Dissertação de Mestrado, Escola de Engenharia Civil, Universidade Federal de Goiás, Goiânia, 122 p.
Pauletti, C. (2004), "Análise comparativa de procedimentos para ensaios acelerados de carbonatação". Dissertação de Mestrado, Universidade Federal do Rio Grande do Sul, Porto Alegre, 176 p.
Pontes, C. V., Réus, G. C., Araújo, E. C., Medeiros, M. H. F. (2020), Silver nitrate colorimetric method to detect chloride penetration in carbonated concrete: how to prevent false positives. Journal of Building Engineering. https://doi.org/10.1016/j.jobe.2020.101860
Real, L. V., Oliveira, D. R. B., Soares, T., Medeiros, M. H. F. (2015), Método colorimétrico por aspersão de nitrato de prata para avaliação da penetração de cloretos em concreto: estado da arte. Revista Alconpat. 5(2): 149-159. https://doi.org/10.21041/ra.v5i2.84
Réus, G. C. (2017), "Realcalinização química como meio de recuperação de estruturas de concreto armado carbonatadas". Dissertação de Mestrado, Pós-graduação em Engenharia de Construção Civil, Universidade Federal do Paraná, Curitiba, 104 p.
Réus, G. C., Medeiros, M. H. F. (2020), Chemical realkalization for carbonated concrete treatment: Alkaline solutions and application methods. Construction and Building Materials, 262, 120880. https://doi.org/10.1016/j.conbuildmat.2020.120880
RILEM. TC 178-TMC - Testing and modelling chloride penetration in concrete. Madrid: Elsevier; 2013. p. 3.
Vennesland, Ø., Climent, M. Á., Andrade, C. (2013). Recommendation of RILEM TC 178-TMC: Testing and modelling chloride penetration in concrete. Materials and Structures. 46: 337-344. https://doi.org/10.1617/s11527-012-9968-1
Slonka-Slupik, B., Podwórny, J., Staszuk, M. (2018), Corrosion of cement pastes made of CEM I and CEM III/A caused by a saturated water solution of ammonium chloride after 4 and 25 days of aggressive immersion. Construction and Building Materials. 170: 279-289. https://doi.org/10.1016/j.conbuildmat.2018.03.073
Yuan, Q., Shi, C., He, F., Schutter, G. D., Audenaert, K., Zheng, K. (2008), Effect of hydroxyl ions on chloride penetration depth measurement using the colorimetric method. Cement and Concrete Research. 38: 1177-1180. https://doi.org/10.1016/j.cemconres.2008.04.003
Wei, Y., Guo, W., Liang, S. (2018), Chloride Ingress in Internally Cured Concrete under Complex Solution . Journal of Materials in Civil Engineering. 30(4) p. 04018037. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002215
04017128 Weiss, J., Couch, J., Pease, B., Laugesen, P., Geiker, M. (2017), Influence of Mechanically Induced Cracking on Chloride Ingress in Concrete. Journal of Materials in Civil Engineering 29(9): . https://doi.org/10.1061/(ASCE)MT.1943-5533.0001922