

Investigación Básica
Apparent diffusion coefficient of oxygen and corrosion control of reinforcement rebar coated with primers
Coeficiente de difusão aparente de oxigênio e o controle da corrosão de armaduras revestidas com primers
Coeficiente de difusión aparente de oxígeno y el control de la corrosión de armaduras revestidas con primers
Apparent diffusion coefficient of oxygen and corrosion control of reinforcement rebar coated with primers
Revista de la Asociación Latinoamericana de Control de Calidad, Patología y Recuperación de la Construcción, vol. 8, no. 3, 2018
Asociación Latinoamericana de Control de Calidad, Patología y Recuperación de la Construcción, A. C.
Received: 13 July 2018
Accepted: 01 August 2018
Published: 31 August 2018
Abstract: The present work evaluates the influence of different primers applied in the reinforcement steel on the apparent diffusion coefficient of oxygen (Dap (O2)) and on the corrosion intensity (Icorr), comparing the results with a reference cimentitious mortar. Oxygen flow (J (O2)) until the reinforcement steel was measured by potenciostático method in steady state. The Icorr was monitored by the Polarization Resistance technique (Rp). Evaluations related porosity of the primers were made through magnifying glasses, optical microscopy and SEM. Primers that represent barrier protection systems proved to be less permeable to oxygen. The Dap (O2) values ranged from 2.1 x 10-6 cm2/s to 4 x 10-9 cm2/s, causing variation in the Icorr due to cathodic control of the corrosion process.
Keywords: reinforced concrete, corrosion control, diffusion of oxygen, primers.
Resumo: O presente trabalho avalia a influência de diferentes revestimentos aplicados nas armaduras sobre o coeficiente de difusão aparente de oxigênio (Dap(O2)) e sobre a intensidade de corrosão (Icorr), comparando os resultados com um revestimento de referência (argamassa cimentícia). O fluxo de oxigênio (J(O2)) até a armadura foi medido pelo método potenciostático no estado estacionário. A Icorr foi monitorada pela técnica de Resistência de Polarização. Avaliações referentes a porosidade dos revestimentos foram feitas por meio de lupas, microscopia ótica e SEM. Os revestimentos que representam sistemas de proteção por barreira mostraram-se menos permeáveis ao oxigênio. Os valores Dap(O2) variaram de 2,1 x 10-6 cm2/s até 4 x 10-9 cm2/s, ocasionando variações na Icorr, devido ao controle catódico do processo de corrosão.
Palavras-chave: concreto armado, controle da corrosão, difusão de oxigênio, primers.
Resumen: El presente trabajo evalúa la influencia de diferentes recubrimientos aplicados en la armadura en el coeficiente de difusión aparente de oxígeno (Dap (O2)) y en la intensidad de corrosión (Icorr), comparando los resultados con un revestimiento de referencia (mortero cimentício). El flujo de oxígeno (J (O2)) hasta la armadura se midió por el método potenciostático en estado estacionario. La Icorr se controló mediante la técnica de resistencia de polarización. Evaluaciones respecto a la porosidad de los recubrimientos fueron hechas con lupas, microscopio óptico y SEM. Los revestimientos que representan sistemas de protección por barrera han resultado menos permeables al oxígeno. Los valores de Dap (O2) variaron de 2.1 x 10-6 cm2/s a 4 x 10-9 cm2/s, causando variaciones en la Icorr debido al control catódico del proceso de corrosión.
Palabras clave: concreto armado, control de la corrosión, difusión de oxígeno, primers.
INTRODUCTION
The rebar of the reinforced concrete structures, typically, are protected from corrosion by a passive layer of oxides formed due the high alkalinity of the concrete, which determines the so-called state of passivation of steel reinforcement. This layer protects indefinitely the steel reinforcement of the corrosion, while the concrete preserve your good quality, no cracks and not have physical or mechanical characteristics changed due to the action of aggressive external agents. The passive protection layer is destabilized by the decrease in the pH of the concrete around the reinforcement to values less than 9, due to the carbonation of concrete, or due the penetration of chloride ions through the porosity of concrete, reaching critical limits, leading to despassivation and start corroding. After the passive layer is broken down and triggered the corrosion process, resistivity and temperature of concrete and the flow of oxygen to the surface of the steel rebar are the main controllers factors of the propagation period of corrosion. (Gjorv; Vennesland; El-Busidy, 1986; Andrade et al., 1990; Castelotte et al., 2001; Francinete; Figueiredo, 1997). The reactions of corrosion can be controlled by several factors, according to illustrate the diagrams of Figure 1. These factors change the polarization characteristics of the reinforcement steel.
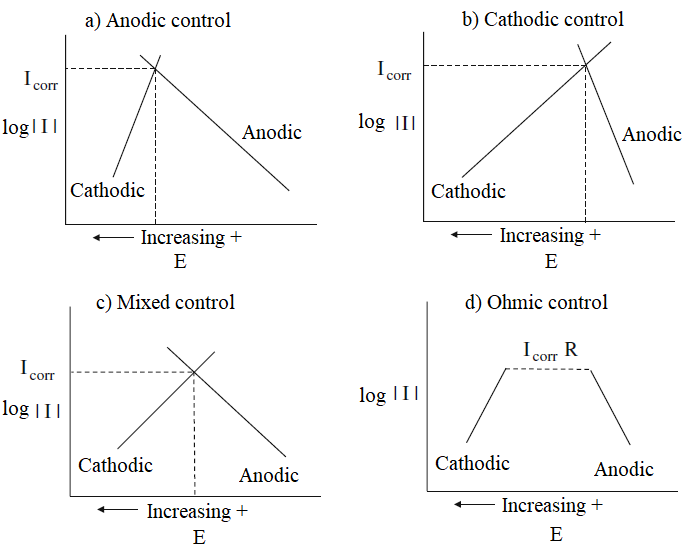
When the polarization occurs mainly at the anode, the corrosion reaction is controlled anodically and the reaction of metal dissolution is diminished. When the resistivity of electrolyte (concrete) is very high, to the point of preventing ion movement, the resulting current is insufficient to polarize the anode and the cathode. In this case, the corrosion reactions are under ohmic resistance control. In practice, the reactions occur in the same intensity at the anode and the cathode and thus has a mixed control. The cathodic control occurs when the oxygen reduction reaction (Equation 1) is constrained by the reduction in oxygen access to the cathodic region, limiting the consumption of electrons from the anode region and, consequently, controlling the kinetics of corrosion.
The presence of oxygen on the surface of the reinforcement steel is essential for reduction reactions occur in the cathodic areas. The oxygen diffusion coefficient in concrete is a concrete property very important and determinant on durability of reinforced concrete structures (Page; Lambert, 1987; Helene, 1993; Hansson, 1993). In some study, the measured oxygen flow is used to predict the durability of the steel reinforcement, based on the relationship between the anodic dissolution, or corrosion, and the amount of oxygen that can be reduced in cathodic areas (Andrade et al., 1990). Kobayashi e Shutton (1991) e Tuutti (1982) studied the influence of the water/cement ratio, the thickness of the covering, the air humidity and the saturation degree of the concrete pores, the presence of mineral additions to cement and concrete curing conditions on the diffusion of oxygen through the concrete.
Restricting the access of oxygen to the steel reinforcement is one of the performance requirements that the coatings applied to the steel reinforcement, or even repair mortars and paints of surface protection, must meet in order to fulfill with efficiency the functions of preservation and restoration of protection and control of reinforcement corrosion.
The measurement of the apparent diffusion coefficient of oxygen (Dap(O2)) through of the concrete covering or through the coatings applied to the steel reinforcement, show the conditions of oxygen supply to the cathodic regions that regulate corrosion kinetics in the anodic regions. Currently, to compose a repair system of concrete structures attacked by corrosion, the technical means has an ample variety of coatings (primers) that are applied on the steel reinforcement. The mechanisms of protection exercised by these coatings can be for barrier, repassivation, inhibition and cathodic protection. In practice usually occurs the joint action of two or more protection mechanisms (Figueiredo, 1994).
The knowledge of the composition and properties of primers (coatings) that are directly related to the ability of protection and control of corrosion is important to the overall assessment of the performance of the primers. Such information is also important for the designers of repairing can choose the products most appropriate for a given situation. Therefore, the present study aims to assess the influence of five different types of coatings, specified for protection of steel reinforcement, on the apparent diffusion coefficient of oxygen (Dap(O2)) and on the intensity of corrosion (Icorr), in comparison to a reference coating composed of a cement-sand mortar.
EXPERIMENTAL PROGRAM
Materials and specimen
For the realization of the experiment were casting prismatic mortar specimens in the dimensions 20 mm x 55 mm x 80 mm. The reference mortar was produced with cement/sand ratio of 1/3 and water/cement ratio of 0.50, both in mass. In the mixing water was mixed 3% CaCl2, in relation to the cement mass, to promote the despassivation of steel reinforcement. The cement used was a high early strength. Table 1 shows the chemical and mineralogical composition and physical and mechanical characteristics of Portland cement used to produce the specimens.
| Chemical Composition | Results (%) |
| CaO | 61,34 |
| SiO2 | 18,32 |
| Al2O3 | 5,43 |
| Fe2O3 | 3,28 |
| SO3 | 3,04 |
| MgO | 1,51 |
| K2O | 1,04 |
| Na2O | 0,15 |
| Cl- | 0,02 |
| P.F. | 3.13 |
| R.I. | 1,92 |
| Mineralogical Composition | Results (%) |
| C3S | 60,54 |
| C2S | 6,85 |
| C4AF | 9,98 |
| C3A | 8,84 |
| Mechanical Characteristics | Results (MPa) |
| Compressive Strength (3 days) | 27,8 |
| Compressive Strength (28 days) | 59,1 |
| Physical Characteristics | Results |
| Initial setting time | 85 minuts |
| Final setting time | 150 minuts |
| Specific weight | 3,15 g/cm3 |
In each specimen were placed two steel reinforcements type CA50 (Brazilian standard denomination) of 6 mm in diameter and 8 cm long, in order to obtain the duplication of results. Table 2 presents the chemical composition of the steel reinforcements used in the experiments.
| Element | Composition (%) |
| Fe (Iron) | 98,94 |
| C (Carbon) | 0,17 |
| Mn (Manganese) | 0,59 |
| Si (Silicon) | 0,25 |
| P (Phosphorus) | 0,02 |
| S (Sulfur) | 0,03 |
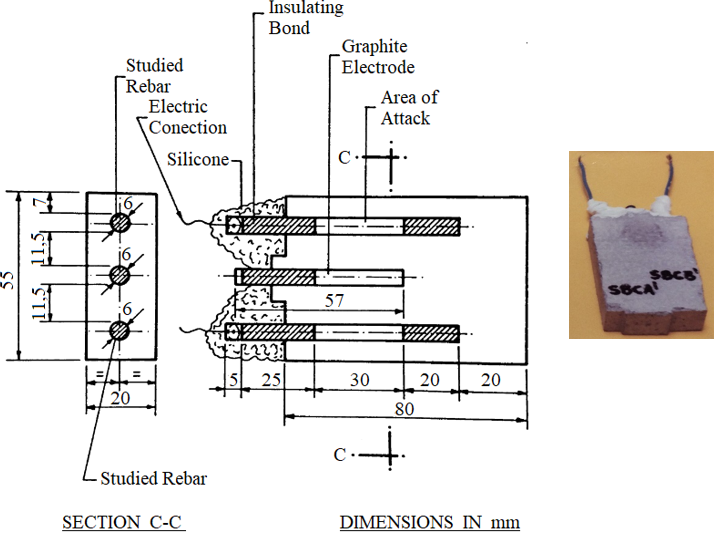
The steel reinforcements (steel bars) went through a cleaning process, in accordance with the ASTM G1 (1999) recommendations, before being immersed in the mortar. This procedure gives to the steel bars identical superficial grade cleaning before being coated. An insulating tape delimited the study area of 5.6 cm2, which was applied protective coating of rebar (primer). Between the two bars of study was placed a graphite bar to act as counter electrode. Figure 2 shows details of the specimen used in the experiments. Table 3 presents the characteristics of coatings (primers) supplied by manufacturers.
| Primer | Composition | Number of Components | Wet thickness (µm) | Density d (kg/l) | pH |
| 1 | Cement + thermoplastic polymer + special loads | 2 | 1000 a 2000 (applied in 2 coats) | 1,90 | > 10 |
| 2 | Cement + thermosetting polymer + inhibitor (Ca(NO2)2) | 3 | 1000 a 2000 (applied in 2 coats) | 2,00 | N.E. |
| 3 | Epoxy + zinc | 1 | 135 µm/demão (applied in 2 coats) | 2,00 | N.E. |
| 4 | Epoxy | 2 | N.E. | N.E. | N.E. |
| 5 | Polymer + lead | 1 | 300 µm (applied in 3 coats) | 1,36 ± 0,05 | 9,4±0,2 |
After casting, the specimens was stored in chamber of 100% relative humidity, remaining in this condition for more than 100 days. In the second stage, until the time of the implementation of the measures, the specimens were provided partially submerged, in order to promote the reinforcement corrosion. The measures of oxygen flow (J (O2)) were made when the specimens completed 1 year.
Experimental methodologies and evaluations
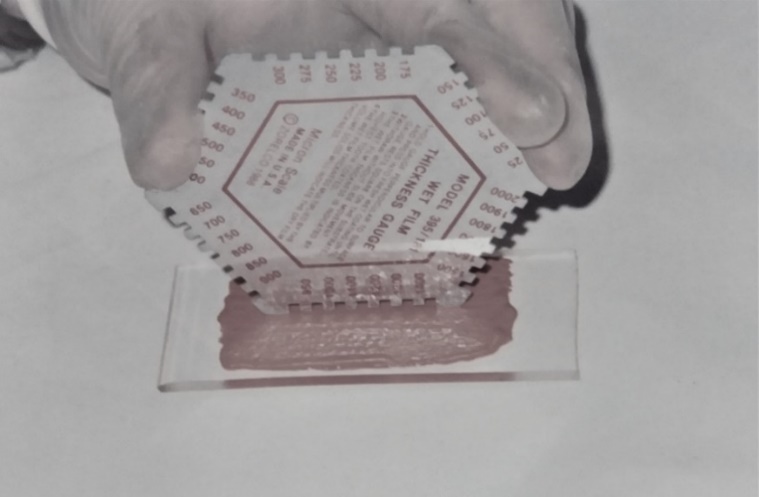
The oxygen flow through a material is influenced by your thick and interconnectivity of your porous network. In this sense, were made measurements of the thickness of each coat and the total thickness of the wet applied coatings (fresh state), employing a fresh film thickness gauge on a glass plate, as shown in Figure 3. The dry thickness of the primers, the estimate of the size of the pores and your interconnectivity were evaluated by means of magnifying glass, optical microscopy and scanning electron microscopy (SEM). The magnifying glass with increased 4 times was employed to identify surface defects of dry films. These evaluations were also important to detect by comparing possible changes existing surface after the end of the tests and rupture of the specimens. When on the surface were detected imperfections, with suspicion that could have continuity and reach the steel reinforcement, made use of the stereomicroscopic to observe and photograph the defects with more details. At various times, to enter through the defect or surface porosity of the second coat, it was possible to identify the presence of primer from the first coat, reaching the conclusion that the pore no continuity. In this sense it is evident the importance of number of coats to the coating adhere your barrier function. Microscopy allowed estimating pore size and the thickness of hardened coatings, identify elements and semi quantitative composition and observe the presence of resin within the porosity, in order to interrupt the continuity of the pores. While the thickness and porosity of coatings are associated with barrier protection mechanism of the steel rebar, the high pH value of coatings is key to activate the repassivation protection mechanism (Figueiredo, 1994). The pH of the coatings was measured with equipment having glass and calomel electrodes combined with pH range 0 to 14. Ph measurements were obtained 15 minutes after mixing of components of coatings.
The corrosion intensity (Icorr) was measured by Polarization Resistance technique, where a polarization of ± 10 mV around of the corrosion potential (Ecorr) was applied and the ohmic drop of the covering was compensated by means of the positive feedback of the potenciostat between the working electrode (reinforcement steel) and the reference electrode (calomel electrode saturated). Intensity changes resulting from the application of potential difference were determined with at a sweep rate of 10mV/min. Corrosion intensity (Icorr) was calculated using the equation of STERN and GEARY (1957).
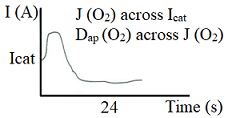
To determine the oxygen flow to the surface of the steel rebar inside of the specimen, was measured the cathodic current (Icat) at a constant potential-750 mV relative to the saturated calomel electrode (ECS). At this level of potential the only reaction possible is the cathodic reduction of oxygen (Gjorv et al., 1986; Andrade et al., 1990). Cathodic intensity (Icat) was measured when the cathodic current versus time curve reached the so-called steady state. After 24 hours of test it was possible to verify that all the reinforcement coated found their stationary states, as shown in Figure 4.
With the value of the Icat as steady-state applied to Faraday's law to get the oxygen flow J(O2)) until the reinforcement steel.
J(O2) -> oxygen flow in mol/second;
Icat -> cathodic current intensity in the steady-state in amper (A);
n -> number of electrons consumed (4);
F -> Faraday constant (96500 coulomb/mol).
From the oxygen flow (J(O2)), and using the first Fick's law, it has been calculated the apparent diffusion coefficient of oxygen (Dap(O2)).
Dap(O2) -> apparent diffusion coefficient of oxygen in cm2/s;
J(O2) -> oxygen flow in mol/s;
e -> thickness of the covering in cm (0,7 cm);
S -> study area in cm2 (5,6 cm2);
Co -> oxygen concentration in a saturated solution of Ca(OH)2 in mol/cm3
(1,06 x 10-6 mol/cm3, in accordance with Page, Lambert, 1987).
RESULTS AND DISCUSSION
Figure 5 shows examples of images obtained by optical and scanning electron microscopy (SEM). During the attainments and evaluations of the images was possible to estimate the thickness and pore size of dry coatings applied on the reinforcement steel, as well as assess the interconnectivity of the pores. The results are in Table 4. In Table 4 are also the thicknesses of the fresh film applied on each coat, total fresh film thickness and the pH of the coatings (primers).

| Primer | Wet thickness (µm) | Dry thickness (µm) | Estimated pore size (µm) | Pore connectivity | pH | |||
| 1ª coat | 2ª coat | 3ª coat | Total | |||||
| Refer | - | - | - | - | 7000 (*) | 1000 (***) | Existence of connectivity | 13,15 |
| 1 | 550 | 550 | - | 1100 | 1000 | ≤ 250 | Frequently interrupted by the presence of resin and overlapping of coats | 12,53 |
| 2 | 700 | 650 | - | 1350 | 800 | ≤ 100 | Frequently interrupted by the overlapping of coats | 11,47 |
| 3 | 175 | 175 | - | 350 | 330 | ≤ 50 | Despite the low porosity, low presence of resin and high zinc allow a lot of connectivity between the pores | 8,48 |
| 4 | 350 | - | - | 350 | 500 (**) | ≤ 40 | Without connectivity | 10,91 |
| 5 | 100 | 100 | 100 | 300 | 250 | ≤ 20 | High presence of small pores with possibility of connections | 8,31 |
The values of cathodic current (Icat), corrosion intensity (Icorr), oxygen flow (J(O2)) and apparent diffusion coefficient of oxygen (Dap(O2)) obtained in experimental evaluation, are presented in Table 5 and Figure 6.
| Primer | Icorr (µA) | Icat (µA) | J(O2) (mol/s) | Dap(O2) (cm2/s) |
| Refer. | 8,000 | 6,800 | 1,76 x 10-11 | 2,07 x 10-6 |
| 1 | 0,004 | 0,030 | 8,00 x 10-13 | 9,00 x 10-9 |
| 2 | 1,200 | 1,500 | 3,90 x 10-12 | 4,60 x 10-7 |
| 3 | 35,000 | 27,990 | 7,25 x 10-11 | 8,55 x 10-6 |
| 4 | 0,008 | 0,013 | 3,00 x 10-13 | 4,00 x 10-9 |
| 5 | 0,250 | 3,010 | 7,80 x 10-12 | 9,20 x 10-7 |
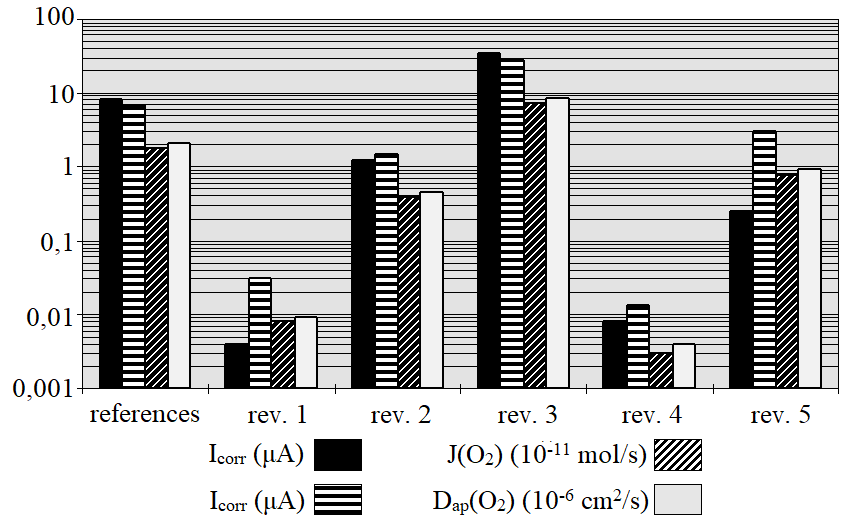
Observing Figure 6 it is possible to note that the less-active rebar, protected with primers 1, 2, 4 and 5, the Icat proved greater than the Icorr. This means that in the reinforcement coated with primers of largest barrier effect the anodic dissolution reactions are controlled, while in the cathodic regions of these rebars, due to introduction of a potential-750 mV (ECS) and the presence of some dissolved oxygen in the vicinity of the rebars, the oxygen reduction reactions end up happening. The reinforcement steel protected with the reference mortar and primer 3, after one year of exposure to chlorides, recorded Icorr values indicative of that were in the process of corrosion. In this case, the high values of Icat registered may indicate that was taking place, also, the reduction of iron oxides presents on the surface of these reinforcements. Figure 6 shows that there are still major differences between the primers studied with regard to its characteristics of permeability to oxygen. The primers 1, 2 and represent barrier protection systems and showed less permeable to oxygen than others under the conditions tested. The assessments regarding the porosity and pore connectivity shown in Table 4 support and help in the understanding of the smallest values of Dap(O2), especially with regard to the primer 4 based in epoxy resin.
The values found for the flow and diffusion coefficient, relative to the reference (cement mortar and sand), were of the same order of magnitude of the found by other authors, as can be seen in Table 6.
| Author | Dap(O2) (cm2/s) |
| Gjorv et al (1986) | 1,3 x 10-6 a 3,4 x 10-6 * |
| Andrade et al (1990) | 2,44 x 10-6 ** |
| Kobayashi et al (1991) | 084 x 10-6 |
| Hansson (1993) | 2,36 x 10-6 ** |
| Figueiredo (1994) | 2,07 x 10-6 |
The area used for the calculation of the Dap (O2), presented in Table 5, was 5.6 cm2, so the total area under study. It is important to note, however, that this may not be correct, since the barrier effect exercised by certain coatings reduces the area that effectively is in contact with the electrolyte, reducing, thus, the area where it would be possible take place the oxygen reduction reaction on the rebar. When there are identical situations (same metal type, same polarization imposed (-750 mV, ECS), same environment (reference cement mortar) and same ambient conditions) for all specimens, it is expected that the oxygen diffusion coefficient calculated is always the same. The differences found, therefore, are probably due to differences in areas where oxygen reduction takes place, which, for your time, depend on the greater or lesser barrier effect exerted by each primer. Based on the above, it is possible deduce the equations 4, 5 and 6 that can be applied to calculate the effective oxygen reduction areas of each case and to compare with the reference.
Drefer (O2) -> is the diffusion coefficient of oxygen of the reference mortar;
Arefer -> is the reference area of the study (5,6 cm 2 );
Dprimer x (O2) -> is the oxygen diffusion coefficient of primer studied.;
Aprimer x -> is the effective area of oxygen reduction related to the primer studied in cm2;
Jrefer -> is the oxygen flow of the reference cement mortar, in mol/s;
Jprimer x -> is the oxygen flow of the studied primer, in mol/s.
Table 7 presents the effective area values (Aprimer x) for each primer studied by using the values of J(O2) presented in Table 6 and applying Equation 6.
| Primer | Refer. | Primer 1 | Primer 2 | Primer 3 | Primer 4 | Primer 5 |
| Aprimer x (cm 2 ) | 5,6 | 0,03 | 1,24 | 23,13 | 0,01 | 2,49 |
The values shown in Table 7 indicate that, with the exception of primer 3, all other exercised at the time of measurement of Icat, barrier effect superior to the reference mortar. The value obtained for the effective area of the primer 3 probably is due to the registration of the reduction of oxygen on the surface of the zinc particles present in this primer.
Due to corrosion of zinc, both Icorr and Icat show higher values than reference because the reactions of anodic oxidation and cathodic reduction get along on the surface of the reinforcement steel and the zinc particles. In this case, the primer 3 would be exercising a cathodic protection mechanism and not by barrier.
The values of J(O2) and Dap(O2) presented in Figure 6, as well as the values of Aprimer x, shown in Table 7 indicate that the primer 4 (epoxy-based polymer coating) represented the largest barrier to the oxygen diffusion.
As time goes by test, primer can deteriorate. Thus, the calculation of the effective area of contact between the electrolyte and the reinforcement steel (Aprimer x) can be used as a parameter to track the evolution of primer deterioration over time, if the area obtained to increase each test.
FINAL CONSIDERATIONS
The primers can protect the reinforcement for passivation, inhibition, cathodic protection and barrier. However, hardly a primer protects the reinforcement steel, along all the time, through a single mechanism. In this study it was found that the majority of primers studied was exercising barrier effect more than reference mortar. The results obtained with the technique employed in this work, show that there are significant differences between the primers with regard to its characteristics of oxygen permeability, and those who represent barrier protection system are less permeable to oxygen under the conditions tested. The value of the oxygen diffusion coefficient obtained in this paper for reference cement mortar are in accordance with the results of other researchers, demonstrating the feasibility of the methodology used to measure the diffusion of oxygen. The electrochemical technique employed in this work allows to track the performance of the primer with the time, watching if there is damage to the primer or not through the monitoring of the effective area of oxygen reduction (Aprimer x) on the reinforcement steel.
REFERENCES
ASTM International. (1999). ASTM G1-90(1999), Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. Retrieved from https://doi.org/10.1520/G0001-90R99E01
Andrade, C.; Alonso, C.; Garcia, M. (1990), Oxygen Availability in the Corrosion of Reinforcements. Advances in Cement Research, v. 3, n. 11, pp. 127-132. https://doi.org/10.1680/adcr.1990.3.11.127
Andrade, C.; Gonzalez, J. A. (1978), Quantitative measurements of corrosion rate of reinforcing steels embedded in concrete using polarization resistance measurements. Werkstoffe und Korrosion. V. 29, p. 515-519. https://doi.org/10.1002/maco.19780290804
Castellote, M.; Alonso, C.; Andrade, C.; Chadbourn, G. A.; Page, C. L. (2001), Oxygen and chloride diffusion in cement pastes as a validation of chloride diffusion coefficients obtained by steady-state migration tests. Elsevier, Cement and Concrete Research. Volume 31, Issue 4, April 2001, Pages 621-625. https://doi.org/10.1016/S0008-8846(01)00469-0
Figueiredo, E. J. P. (1994) Avaliação do Desempenho de Revestimentos para Proteção da Armadura Contra a Corrosão Através de Técnicas Eletroquímicas: Contribuição ao Estudo de Reparo de Estruturas de Concreto Armado. São Paulo, EPUSP, \Tese de Doutorado\, 423p.
Francinete, P. J; Figueiredo, E. J. P. (1999), Estudo da Difusão de Oxigênio no Concreto. São Paulo. EPUSP, BT/PCC/238, ISSN 0103-9830, 22p.
Gjørv, O.; Vennesland, O.; El-Basaidy, A. H. S. (1986), Diffusion of Dissolved Oxygen through Concrete. Materials Performance, v. 25, pp. 39-44.
Hansson, C. M. (1993), Oxygen Diffusion Through Portland Cement Mortars, Corrosion Science, vol. 35, n. 5-8, pp. 1551 – 1556. https://doi.org/10.1016/0010-938X(93)90383-R
Helene, P. R. L. (1993), Contribuição ao Estudo da Corrosão em Armaduras de Concreto Armado. São Paulo, CPGECC/EPUSP, \Tese de Livre Docência\.
Kobayashi, K.; Shutton, K. (1991), Oxygen Diffusivity of Various Materials. Cement and Concrete
McCafferty, E. (2010), Kinetics of Corrosion. In: Introduction to Corrosion Science. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-0455-3
Page, C.; Lambert, P. (1987), Kinetics of Oxygen Diffusion in Hardened Cement Pastes. Journal of Materials Science, v. 22, pp. 942-946.
Stern, M.; Geary, A. L. (1957), Electrochemical Polarization. A theorical Analysis of the Sharp of Polarization Curves. Journal Electrochemical Society, Vol. 104, nº 1, pp 56-63.
Tuutti, K. (1982), Corrosion of steel in concrete. Sweden: CBI, 468 p.
Additional information
Cite as: E. Pazini Figueiredo, C. Andrade (2018), " Apparent diffusion coefficient of oxygen and
corrosion control of reinforcement rebar coated with primers ", Revista
ALCONPAT, 8 (3), pp. 288-300, DOI: http://dx.doi.org/10.21041/ra.v8i3.336
Legal Information: Revista ALCONPAT is a quarterly publication by the Asociación Latinoamericana de Control de Calidad,
Patología y Recuperación de la Construcción, Internacional, A.C., Km. 6 antigua
carretera a Progreso, Mérida, Yucatán, 97310, Tel.5219997385893, alconpat.int@gmail.com,
Website: www.alconpat.org
Responsible editor: Pedro
Castro Borges, Ph.D. Reservation of rights for exclusive use
No.04-2013-011717330300-203, and ISSN 2007-6835, both granted by the Instituto
Nacional de Derecho de Autor. Responsible for the last update of this issue,
Informatics Unit ALCONPAT, Elizabeth Sabido Maldonado, Km. 6, antigua carretera a Progreso, Mérida, Yucatán, C.P. 97310.
The views of the authors do
not necessarily reflect the position of the editor.
The total or partial
reproduction of the contents and images of the publication is strictly
prohibited without the previous authorization of ALCONPAT Internacional
A.C.
Any dispute, including the
replies of the authors, will be published in the second issue of 2019 provided
that the information is received before the closing of the first issue of 2019.